58 for the ibrutinib group versus 10 for standard therapy p0003. No major bleeding events or.
 Long Term Efficacy And Safety Of Ibrutinib For Chronic Lymphocytic Eha Library Rogovaia I Jun 12 2020 297840
Long Term Efficacy And Safety Of Ibrutinib For Chronic Lymphocytic Eha Library Rogovaia I Jun 12 2020 297840
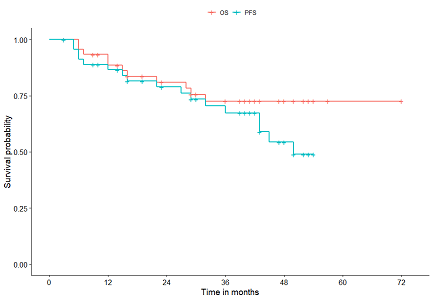
For the overall population median progression-free survival PFS was 196 months 95 CI 165-243 while median overall survival OS was 258 months 95 CI.

Ibrutinib survival rates. NCT01578707 391 patients with relapsed or refractory CLL or small. In the frontline setting the median progression-free survival PFS and median overall survival OS were still not yet met. 56 In 30 patients with relapsedrefractory RR CLL treated with ibrutinib and bendamustine hydrochloride plus rituximab the CR rate.
At 12 months of follow-up 90 percent of patients in the ibrutinib group were still alive compared with 81 percent of patients in the ofatumumab group. Thus patients who received ibrutinib had a 78 percent reduction in the risk of disease progression or death compared with those in the ofatumumab group. Extended treatment with ibrutinib leads to an increase in the CR rate over time.
Twenty-four percent discontinued ibrutinib at 138 months median follow-up. P 0001 and 63 high risk 83 intermediate and 93 low risk. P value not reported.
The 30-month overall survival rate was similar in both treatment arms 94 vs. This analysis included the 30 patients in the placebo arm who were allowed to cross over to single-agent ibrutinib. 10 An important finding was the identification of MYD88 and.
In this study ibrutinib was highly active with an overall response rate ORR of 91 major response rate MRR of 73 and estimated 2-year progression free survival PFS and overall survival OS of 69 and 95 respectively. According to the study results IMBRUVICA was associated with significantly longer progression free survival PFS. The 7-year PFS rate in this setting was 80 and the OS rate was 75.
The median duration of treatment was higher in those who received ibrutinib in the third-line versus other lines 149. Extended treatment with ibrutinib may be feasible in patients with relapsed or refractory chronic lymphocytic leukemia CLL according to research presented at the 2019 American Society of Clinical Oncology ASCO Annual Meeting in Chicago Illinois. In the phase 3 RESONATE trial ClinicalTrialsgov Identifier.
The results of a trial on 391 patients showed the drug Ibrutinib gave patients fighting a type of slow growing blood cancer called Chronic lymphocytic leukaemia CLL a 90 per cent chance of. 95 CI 024 to 079. Four patients died in the ibrutinib group and.
Patients with ZAP-70 expressed on 20 or more of B cells have a median survival of 6 to 10 years compared with more than 15 years for patients with no or minimal expression of ZAP-70. 8 Continued durable activity of ibrutinib in these patients was recently reported with an estimated 5-year PFS of 54. Ibrutinib Imbruvica as a frontline treatment led to sustained efficacy and impressive 4-year progression-free and overall survival OS rates in patients with chronic lymphocytic leukemia CLL.
3-5 In 31 patients with CLL in the first-line setting who received single-agent ibrutinib the CR rate increased from 13 at primary analysis with a median follow-up of 221 months interquartile range 184-232 to 29 with an extended follow-up of approximately 5 years. 47 high risk 74 intermediate and 87 low risk. Ibrutinib is effective in the routine clinical care of both treatment.
The 1-year progression-free survival rate for the entire cohort was 24. CXCR4 mutations impacted response and survival outcomes to ibrutinib monotherapy. Ibrutinib Improves Survival Rates in CLL with Del17p In updated analysis PFSOS surpassed those for all other existing therapies for such patients by Neil Osterweil Contributing Writer.
Toxicity was the most common reason for discontinuation though progression andor transformation accounted for a larger proportion of discontinuations in rates were similar for. The 3-year progression-free survival and overall survival rates were worse at higher scales across the cohorts. 59 percent in RR CLLSLL with median follow-up of 44 months including in patient subgroups with genomic abnormalities that.
The 4-year rates of ibrutinib discontinuation in ON and OFF trial patients were 36 and 44 respectively p 011. Fewer than 5 of all patients on ibrutinib monotherapy achieve a CR although higher CR rates have been reported with prolonged use of ibrutinib. Ibrutinib as compared with ofatumumab significantly prolonged the rate of overall survival hazard ratio for death in the ibrutinib group 043.