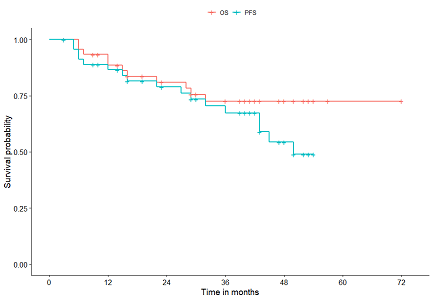
Median OS 95 confidence interval CI was 217 months 180-317 with axitinib versus 233 months 181-332 with sorafenib stratified HR 0995. Correlative analyses demonstrated that PI3K signaling pathway alterations were associated with an increased response to therapy 75 vs 17.
 Kaplan Meier Curve Of Progression Free Survival Of Axitinib Versus Download Scientific Diagram
Kaplan Meier Curve Of Progression Free Survival Of Axitinib Versus Download Scientific Diagram
The best overall response rate was 42.

Axitinib overall survival. In the current updated analysis axitinib did not prolong survival compared with sorafenib but clinical activity of axitinib in patients with previously untreated mRCC was confirmed and its safety after long-term treatment was shown. Axitinib and sunitinib are antiangiogenic medicines that can block the growth of blood vessels to the tumor therefore limiting its growth. This analysis evaluated overall survival OS and safety in 44 Japanese patients and compared the results with.
Median overall survival was 201 months 95 CI 167-234 with axitinib and 192 months 175-223 with sorafenib hazard ratio HR 0969 95 CI 0. Demonstrating superior overall survival OS versus sunitinib in this population8. Sixty-four Japanese patients with metastatic renal cell carcinoma following prior therapy with cytokines were treated with axitinib at a starting dose of 5 mg bid.
Progression-free survival with axitinib and overall survival Median PFS with axitinib treatment was 135 months 95 confidence interval CI 20251 for all patients evaluated. The 5-year survival rate by American Joint Committee on Cancer AJCC tumour lymph nodes and metastasis TNM staging is 8 for Stage IV RCC 3. It is estimated there will be 73820 new cases and 14770 deaths from kidney cancer in the United States in 2019 about 95 of which result from renal cell cancers.
Median overall survival 95 confidence interval CI was 427 months 247-not estimable with axitinib titration versus 304 months 237-450 with placebo titration stratified hazard ratio 0785. 1 Metastatic RCC has a 5-year survival rate of 12. Oncological outcomes and safety were compared between axitinib n 68 and sunitinib n 101 groups.
The median overall survival of 98 months and the 6month overall survival rate of 70 met the protocoldefined criteria for clinical efficacy. 15-255 and 92 range. There was also a statistically significant 37 -month higher investigator-assessed progression-free survival in the axitinib group HR 064 95 CI.
Axitinib-administered group were nausea 1 and diarrhea 1. 1-sided P 162 and 416 months. Following median treatment duration of 142 months median overall survival was 373 months 95 CI 286499.
1-sided P 4883. 1 shows the Kaplan-Meier survival curves for the OS of patients administered with nivolumab or axitinib as second-line treatment. Inverse probability of treatment weighted IPTW-adjusted Cox regression analysis was performed to evaluate effects of first-line therapies on progression-free survival PFS cancer-specific survival CSS and overall survival OS.
The objective of the present study is to evaluate progression-free survival PFS and overall survival OS of cabozantinib compared to everolimus nivolumab axitinib sorafenib and best supportive care BSC in aRCC patients who progressed. In May 2019 the combination of avelu-mab a programmed death-ligand1 PD-L1 inhibitor with axitinib a VEGF receptor TKI was approved as first-line treat-ment for advanced RCC after showing significantly extended. There was no significant difference in PFS between the patient groups with and without UPI 2 during axitinib treatment Fig.
Overall survival and biomarker analysis of a phase Ib combination study of toripalimab a humanized IgG4 mAb against programmed death-1 PD-1 with axitinib. Subgroup analyses of a randomized global phase II study of axitinib showed objective response rate of 66 and median progressionfree survival of 276 months in treatmentnaïve Japanese patients with metastatic renal cell carcinoma RCC. The treatment landscape for patients with mRCC has evolved substantially in the past decade with the introduction of targeted therapies which has led to significant improvements in patient outcomes.
Survival of 121 months compared with 65 months in the sorafenib group HR 046 95 CI 032 to 068 p. The median OS for nivolumab- n9 and axitinib-administered patients n16 was 123 range. 5 rijen Median overall survival was 201 months 95 CI 167234 with axitinib and 192 months.