Other companies in the hunt with a BCMA CAR-T therapies include Johnson Johnson and Novartis. Mit Tisagenlecleucel Kymriah und Axicabtagen-Ciloleucel Yescarta sind bereits zwei CAR-T-Zelltherapeutika im Markt sie sind aber nicht beim Multiplen Myelom zugelassen.
 Car T Cell Therapy Research Tools Sino Biological Bio Connect
Car T Cell Therapy Research Tools Sino Biological Bio Connect
Our goal was to determine if BCMA is a suitable target for CAR-expressing T cells.

Novartis bcma car t. B-cell maturation antigen BCMA is a rational target in multiple myeloma MM. Initially investigated in multiple global phase 2 trials Novartis CAR-T cell therapy research will continue to expand its international reach. Chimeric antigen receptor CAR T cells are a promising therapy for hematologic malignancies.
Novartis is pioneering the way in the class of cell and gene treatment and has developed the first CAR-T cell therapy approved for paediatric and young adult patients with relapsedrefractory B-cell acute lymphoblastic leukaemia ALL. BCMA targeting chimeric antigen receptor CAR T cell therapy has shown deep and durable responses in multiple myeloma. 2-5 Novartis is committed to building on this innovation in CAR-T cell therapy and beyond researching its CAR-T cell therapy in.
CAR T cells are a promising therapy for hematologic malignancies. 111-16 Novartis will continue to add new treatment centre locations collaborating with an increasing number of hospitals to. B cell maturation antigen BCMA a plasma cell surface antigen and member of the tumor necrosis factor TNF receptor superfamily is an attractive target for immunotherapy of multiple myeloma due to its high prevalence on malignant plasma cells.
Using an anti-BCMA CAR T we demonstrated that lenalidomide enhances CAR T cell function in a concentration-dependent manner. 2 This treatment is currently approved in Australia Canada the EU Israel Japan Switzerland Hong Kong and the US. Since 2012 Novartis has partnered with the University of Pennsylvania leading to the first approved CAR-T cell therapy in any disease state.
Autologous HSCT within 6 weeks prior to enrollment or any prior history of allogeneic hematopoietic stem cell transplant HSCT. However relapse following therapy is frequently observed and mechanisms of resistance remain ill-defined. The application of nanobodies in CAR-T therapy has been well demonstrated from bench to bedside and displays great potential in forming advanced CAR-T for more challenging tasks.
The FDA based its. Developing the future of CAR-T cell therapy today. Now the Swiss company which.
The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Amgen is also developing an antibody drug that targets BCMA. Here we perform single cell genomic characterization of longitudinal samples from a patient who relapsed after initial CAR T cell treatment with lack of.
1 This treatment is now approved in two indications across four continentsAsia Australia Europe and North America. Patients who have received prior BCMA-directed bi-specific antibodies or antibody-drug conjugates ADC are not excluded. Bei dieser Therapie kommen gentechnologisch veränderte T-Zellen.
BCMA-directed CAR-T Cell Therapy in Adult Patients With Relapsed andor Refractory Multiple Myeloma. A team at the Novartis Institutes for BioMedical Research is working with Penn on internal CAR-T programs a company spokesperson said. Listing a study does not mean it has been evaluated by the US.
Die Bekämpfung von Tumoren die für das Immunsystem unsichtbar sind. We conducted a phase I study of autologous T cells lentivirally-transduced with a fully-human BCMA-specific CAR containing CD3ζ and 4-1BB signaling domains CART-BCMA in subjects with relapsed. CAR T cells directed against the B-cell maturation antigen BCMA have demonstrated impressive initial results but available data suggest that most patients with initial responses eventually progress.
Know the risks and potential benefits of clinical studies and talk to your health care provider. B cell maturation antigen BCMA is a rational target in multiple myeloma MM. CAR-T-Zellen lösen ein zentrales Problem der Krebstherapie.
Lenalidomide increased CAR T effector cytokine production particularly under low CAR stimulation or in the presence of inhibitory ligand programmed cell death 1 ligand 1. Die Bindung der CAR-T-Zellen an BCMA führt zur Aktivierung der Immunzellen und schließlich zur Zytokin-Sekretion und zum zytolytischen Abtöten der BCMA-exprimierenden Zellen. Poseida gets Novartis backing for myleoma CAR-T challenger San Diego firm joins crowded field Gene-engineering specialist Poseida Therapeutics is launching its own challenger into the crowded CAR-T pipeline for multiple myeloma and has just gained backing from Novartis.
That collaboration is ongoing. 1- 4 New strategies are therefore needed to improve CAR T-cell. This heritage includes setting the standard in patient and caregiver support safety and efficacy access.
We conducted an assessment of BCMA expression in normal human tissues and multiple myeloma cells by flow cytometry quantitative PCR and immunohistochemistry. We designed and tested novel anti-BCMA CARs. Interestingly anti-BCMA CAR-T modulated by a single nanobody or bi-valent nanobody displays comparable clinical effects with that of single-chain variable fragment scFv-modulated CAR-T.
Prior administration of a genetically modified cellular product including prior BCMA CAR-T therapy. Notably lenalidomide also enhanced CAR T cytokine production cytolytic activity and.
Http Plan Medone Co Kr 70 Icksh2019 Data Ss03 2 Jesus Berdeja Pdf
Https Www Ema Europa Eu En Documents Presentation Presentation Chimeric Antigen Receptor Car T Cells David Lebwohl En Pdf
 Current Challenges And Emerging Opportunities Of Car T Cell Therapies Sciencedirect
Current Challenges And Emerging Opportunities Of Car T Cell Therapies Sciencedirect
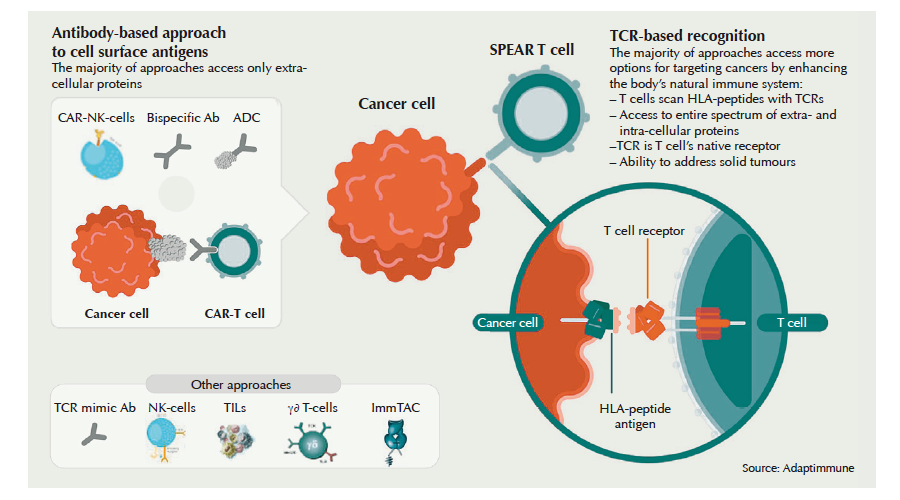 Cars Right On Track European Biotechnology
Cars Right On Track European Biotechnology
Poseida Gets Novartis Backing For Myleoma Car T Challenger Pmlive
 Novartis And Gilead S Multiple Myeloma Cars Diverge Evaluate
Novartis And Gilead S Multiple Myeloma Cars Diverge Evaluate
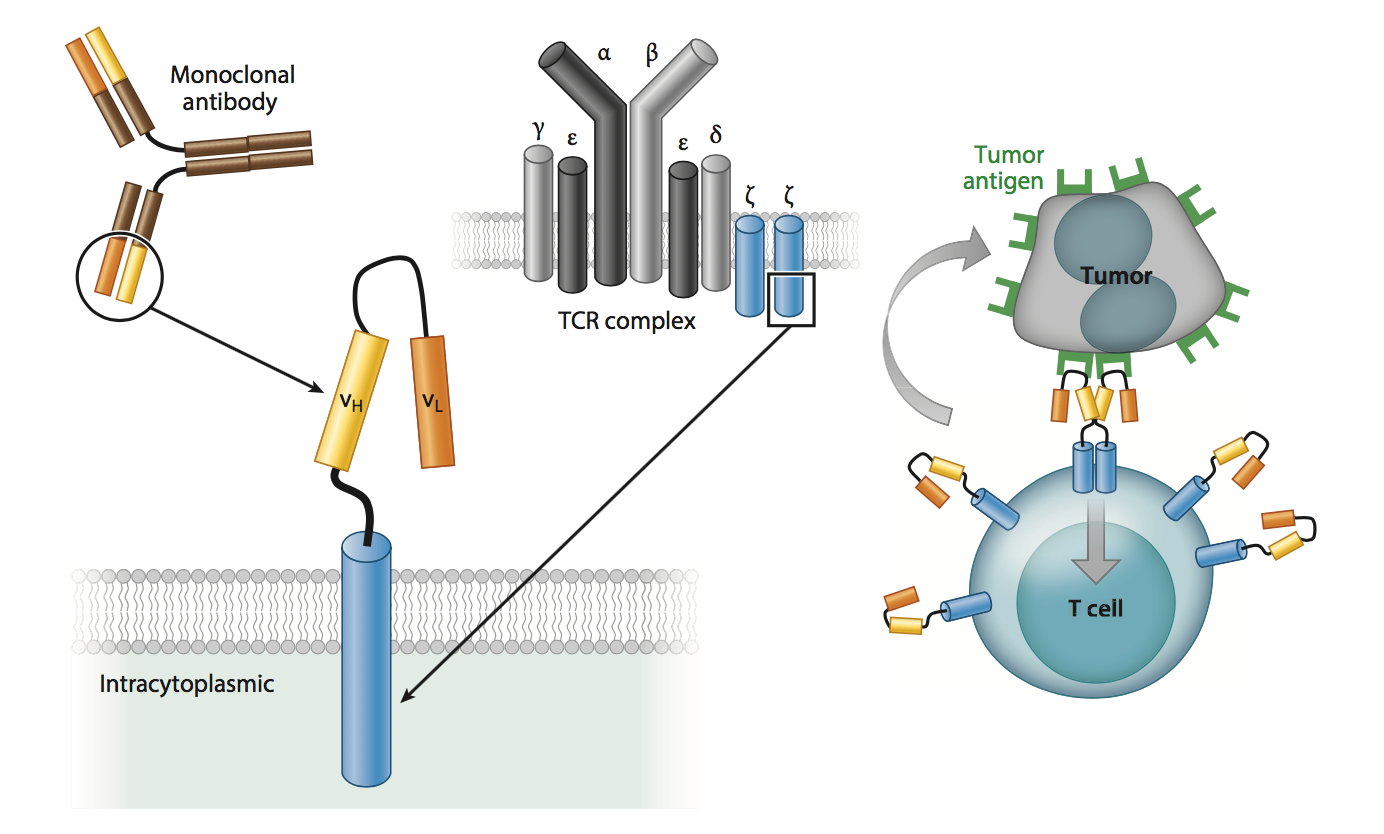 The I O Movement Priming The Immune System To Fight Cancer Part I Chimeric Antigen Receptor T Car T Cell Technology Dilworth Ip
The I O Movement Priming The Immune System To Fight Cancer Part I Chimeric Antigen Receptor T Car T Cell Technology Dilworth Ip
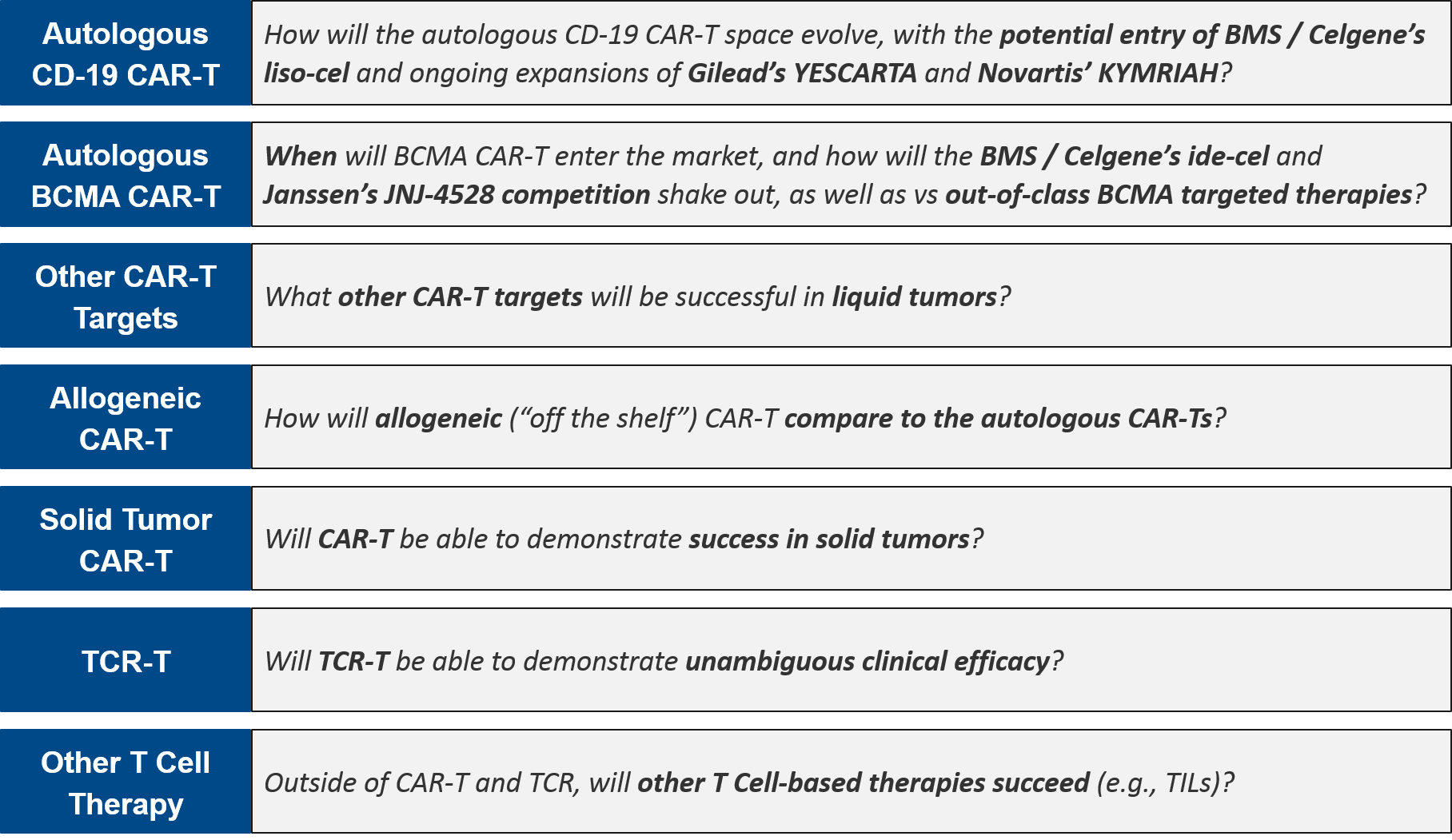 T Cell Therapies Mid 2020 Update Blue Matter Consulting
T Cell Therapies Mid 2020 Update Blue Matter Consulting
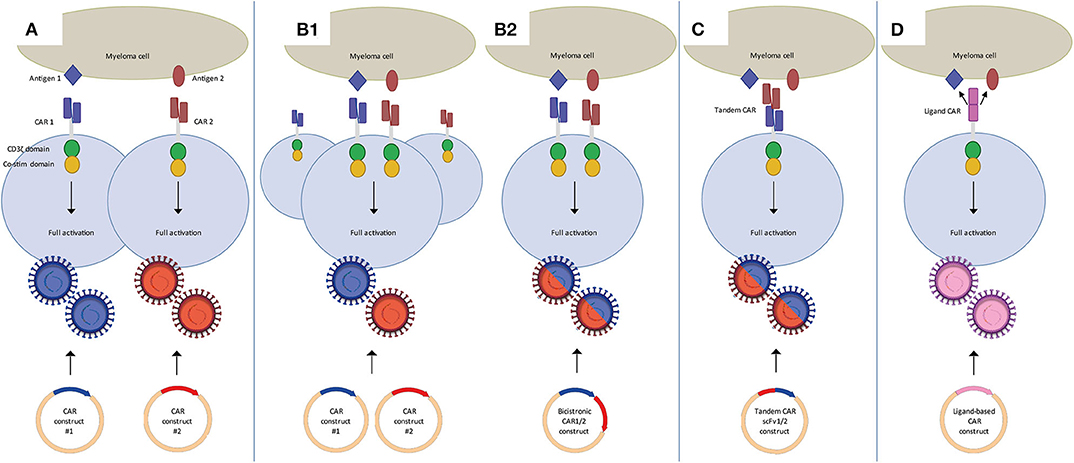 Frontiers Dual Targeting To Overcome Current Challenges In Multiple Myeloma Car T Cell Treatment Oncology
Frontiers Dual Targeting To Overcome Current Challenges In Multiple Myeloma Car T Cell Treatment Oncology
 B Cell Maturation Antigen Bcma In Multiple Myeloma Rationale For Targeting And Current Therapeutic Approaches Leukemia
B Cell Maturation Antigen Bcma In Multiple Myeloma Rationale For Targeting And Current Therapeutic Approaches Leukemia
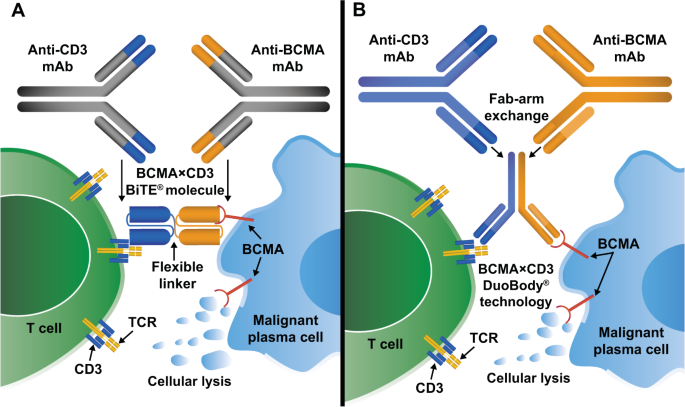 B Cell Maturation Antigen Bcma In Multiple Myeloma Rationale For Targeting And Current Therapeutic Approaches Leukemia
B Cell Maturation Antigen Bcma In Multiple Myeloma Rationale For Targeting And Current Therapeutic Approaches Leukemia
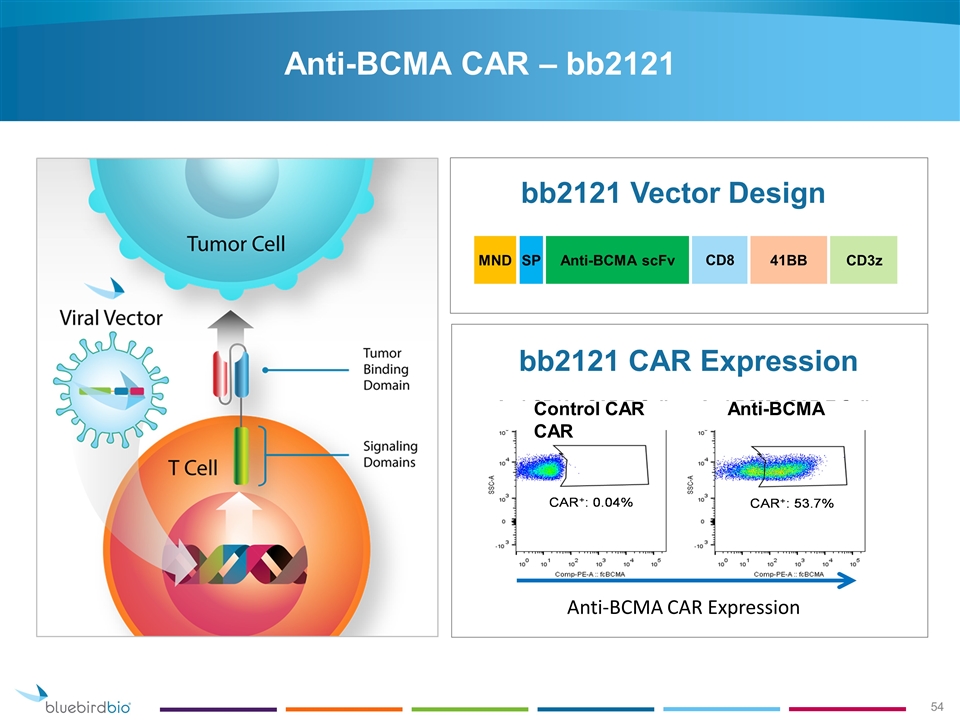 Car T Celtherapie Met Het B Cell Maturation Antigen Bcma Geeft Alsnog Complete Remissies Bij Meer Dan De Helft Van De 18 Geselecteerde Zwaar Voorbehandelde Patienten Met Vergevorderde Multiple Myeloma Kahler
Car T Celtherapie Met Het B Cell Maturation Antigen Bcma Geeft Alsnog Complete Remissies Bij Meer Dan De Helft Van De 18 Geselecteerde Zwaar Voorbehandelde Patienten Met Vergevorderde Multiple Myeloma Kahler
 The Pioneers Of Car T Cell And Gene Therapy Novartis
The Pioneers Of Car T Cell And Gene Therapy Novartis
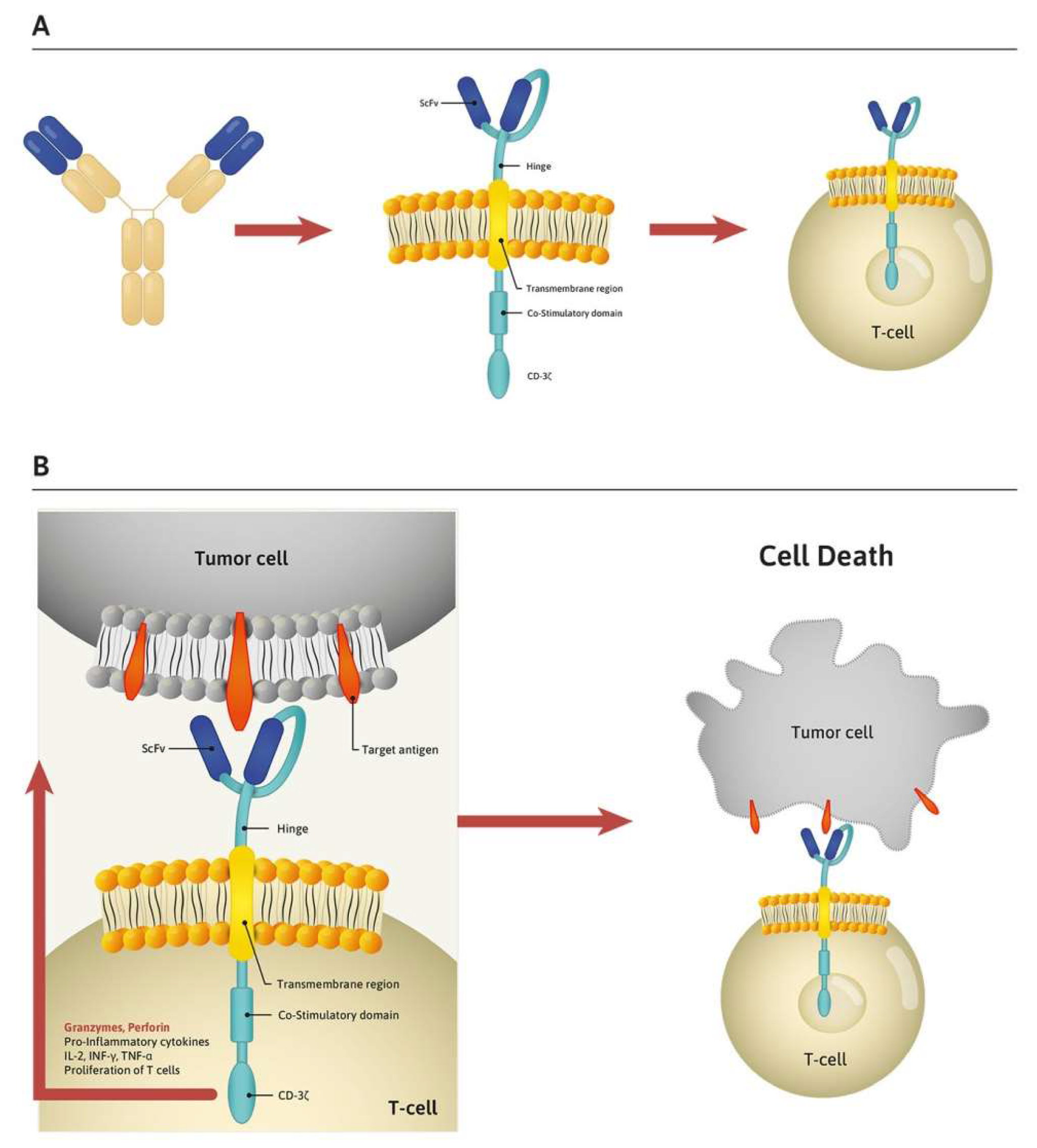 Cells Free Full Text Paving The Way Toward Successful Multiple Myeloma Treatment Chimeric Antigen Receptor T Cell Therapy Html
Cells Free Full Text Paving The Way Toward Successful Multiple Myeloma Treatment Chimeric Antigen Receptor T Cell Therapy Html
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.