The sTMS device is. Food and Drug Administration today approved Ubrelvy ubrogepant tablets for the acute immediate treatment of migraine with or without aura a sensory phenomenon or.
 Neurolief Gets Ce Mark For Digital Migraine Treatment Device
Neurolief Gets Ce Mark For Digital Migraine Treatment Device
TUESDAY March 11 2014 HealthDay News -- The US.

New migraine treatment device. May 29 2019 -- The FDA has cleared a noninvasive device to relieve acute migraine pain. CEFALY is an External Trigeminal Nerve Stimulation device e-TNS that sends tiny electrical impulses through an electrode positioned on the forehead to modify pain transmission and processing in the trigeminal nerve. The Transcutaneous Vagus Nerve Stimulator is a noninvasive handheld tool that uses electrical stimulation to target the vagus nerve in the neck.
In early March 2014 the FDA approved for use a transcutaneous electrical nerve stimulation device for the prevention of migraine known as Cefaly. FDA approvals as of this date include. This type of device would target the branch of the trigeminal nerve.
4 rows Nerivio is approved for acute migraine treatment in adults who do not have chronic migraine. December 23 2019 The US. A Smartphone-Controlled Device to Ease Migraine Pain Some of the devices on the market today include Cefaly electroCores GammaCore and eNeura sTMS This device is not currently available.
As it stimulates it. Erenumab Aimovig is the first medicine specifically approved to prevent migraine attacks. In clinical trials people.
T oday electroCore LLC announced that its neuromodulation device gammaCore has received clearance from the US FDA for acute Migraine treatment in adults. What Kind Of Migraine Devices Are There For Acute Treatment. The Nerivio neuromodulation device from Theranica is now approved for acute treatment of migraine attacks in people aged 12 and older with episodic and chronic migraine disease10 Nerivio is an armband controlled by a mobile phone app available for iPhone and Android.
Nerivio Migra is a first-in-category product according to Theranica the company that makes it. Since the FDA approval many news outlets have been reporting on the availability of the device and many patients have been inquiring about the efficacy of the device and whether it is right for them. Nerivio is used for the acute treatment of migraine attacks.
2 out of 3 People Experienced Pain Relief With New Device. This stimulation is targeted towards the vagus nerve. Migraines have a big impact on the overall quality of a.
FDA Greenlights New Wearable Device for the Treatment of Acute Migraine Migraine. The neuromodulation device was first approved in 2014 by the FDA for acute treatment of Migraine and received the CE mark in Europe for acute and preventive treatment in 2014 and 2016 respectively. The vagus nerve stimulation device was first approved by the FDA in April 2017 for the acute treatment of episodic cluster headaches.
It is a device which is attached to the upper arm and controlled via a smartphone. Single Pulse Transcranial Magnetic Stimulator sTMS. The device called Cefaly is.
A single device CEFALY puts control in the users hands with two program settings ACUTE PREVENT for migraine pain relief. Nerve Stimulation Medical Devices for Migraine Headache External Trigeminal Nerve Stimulation e-TNS. Food and Drug Administration on Tuesday approved the first device aimed at preventing migraines.
To use the device patients place it on the side of the neck over the cervical branch of the vagus nerve until small muscle contractions are felt under the skin. Nerivio uses electrical signals to calm down neurons during a migraine attack. The vagus extends from the.
Essentially it can interfere with pain signals communicated to the brain. The device can be used to treat acute migraine attacks and as prophylaxis between attacks. The small handheld device is about the size of a mobile phone.
In February 2021 the FDA approved Relivion a noninvasive neuromodulation device for the treatment of acute Migraine in adults. Sold under the brand name Cefaly the e-TNS device is based on a. A Lifelong Disorder and Quality-of-Life Concern.
With this new 2019 FDA clearance the sTMS is now the only migraine product in the United States indicated both for the. FDA approval was based on the results of a. You give yourself an injection once a month with a pen-like device.
In 2017 the FDA approval expanded to include preventive use in adults. It has been approved for the acute treatment of attacks in patients with episodic cluster headache as well as for the acute treatment of migraine pain.
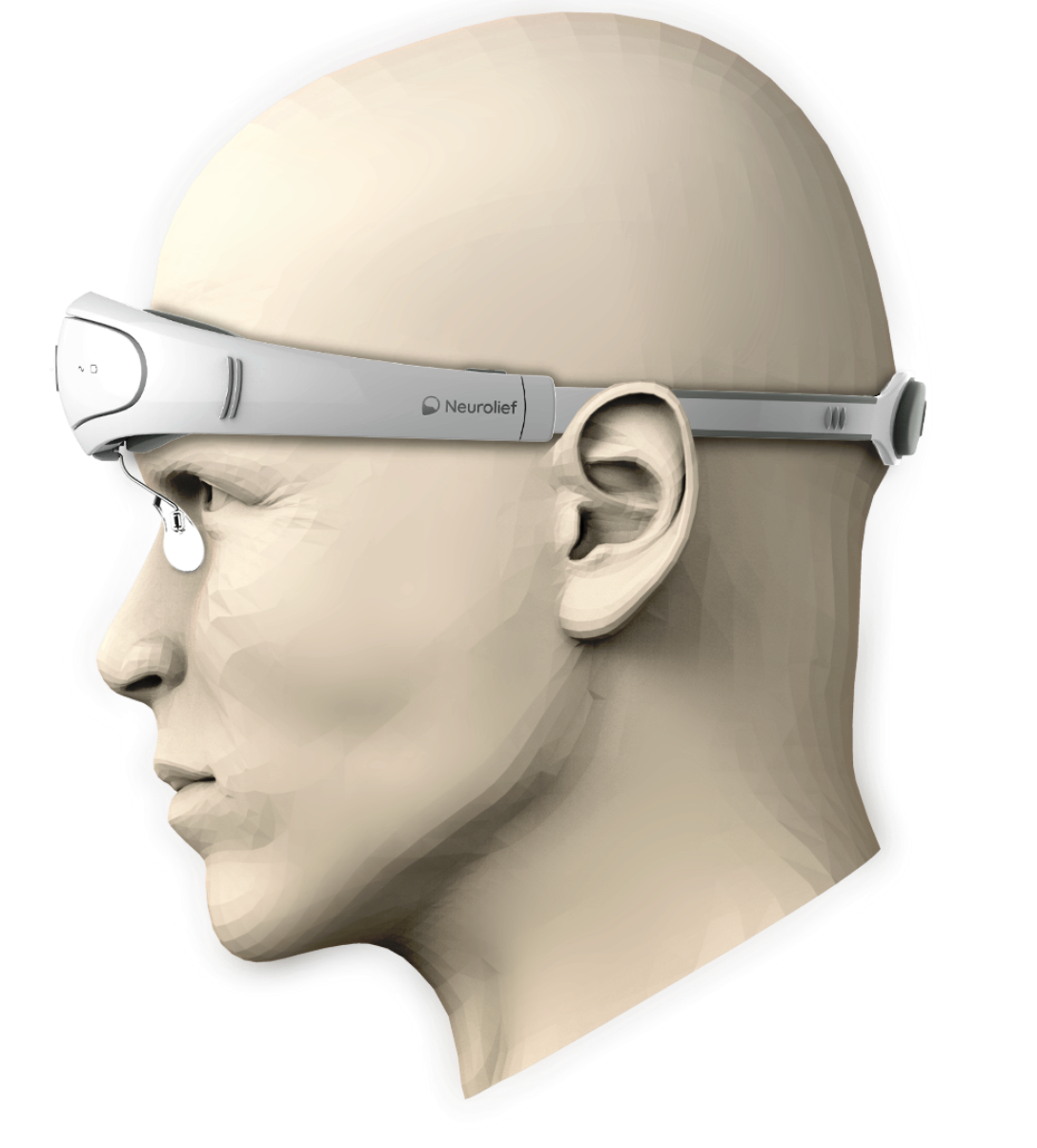 Migraine Relief From An Israeli Neuro Modulation Device Israel21c
Migraine Relief From An Israeli Neuro Modulation Device Israel21c
 Positive Results Published On The Acute Treatment Of Migraine Using The Cefaly Medical Device
Positive Results Published On The Acute Treatment Of Migraine Using The Cefaly Medical Device
 Now Over The Counter Fda Clears Cefaly Dual Migraine Treatment For Use Without A Prescription
Now Over The Counter Fda Clears Cefaly Dual Migraine Treatment For Use Without A Prescription
 Two New Migraine Treatment Devices Available Mpr
Two New Migraine Treatment Devices Available Mpr
 Electronic Headband Prevents Migraines With Tiny Jolts Shots Health News Npr
Electronic Headband Prevents Migraines With Tiny Jolts Shots Health News Npr
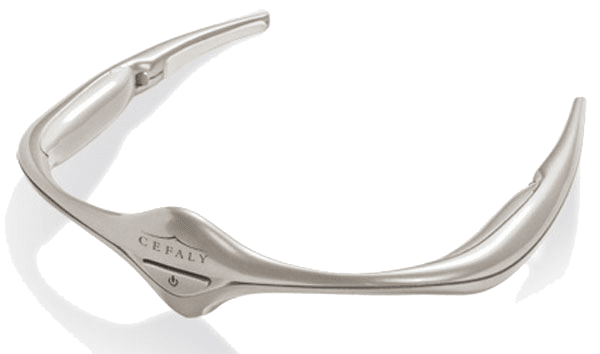 Cefaly Review For Migraine Does It Work Migrainepal
Cefaly Review For Migraine Does It Work Migrainepal
 Migraine Medical Device Cefaly Releases Revamped Pocket Sized Model Of Fda Approved Headband
Migraine Medical Device Cefaly Releases Revamped Pocket Sized Model Of Fda Approved Headband
 Fda Approves Device To Treat Migraines With Auras
Fda Approves Device To Treat Migraines With Auras
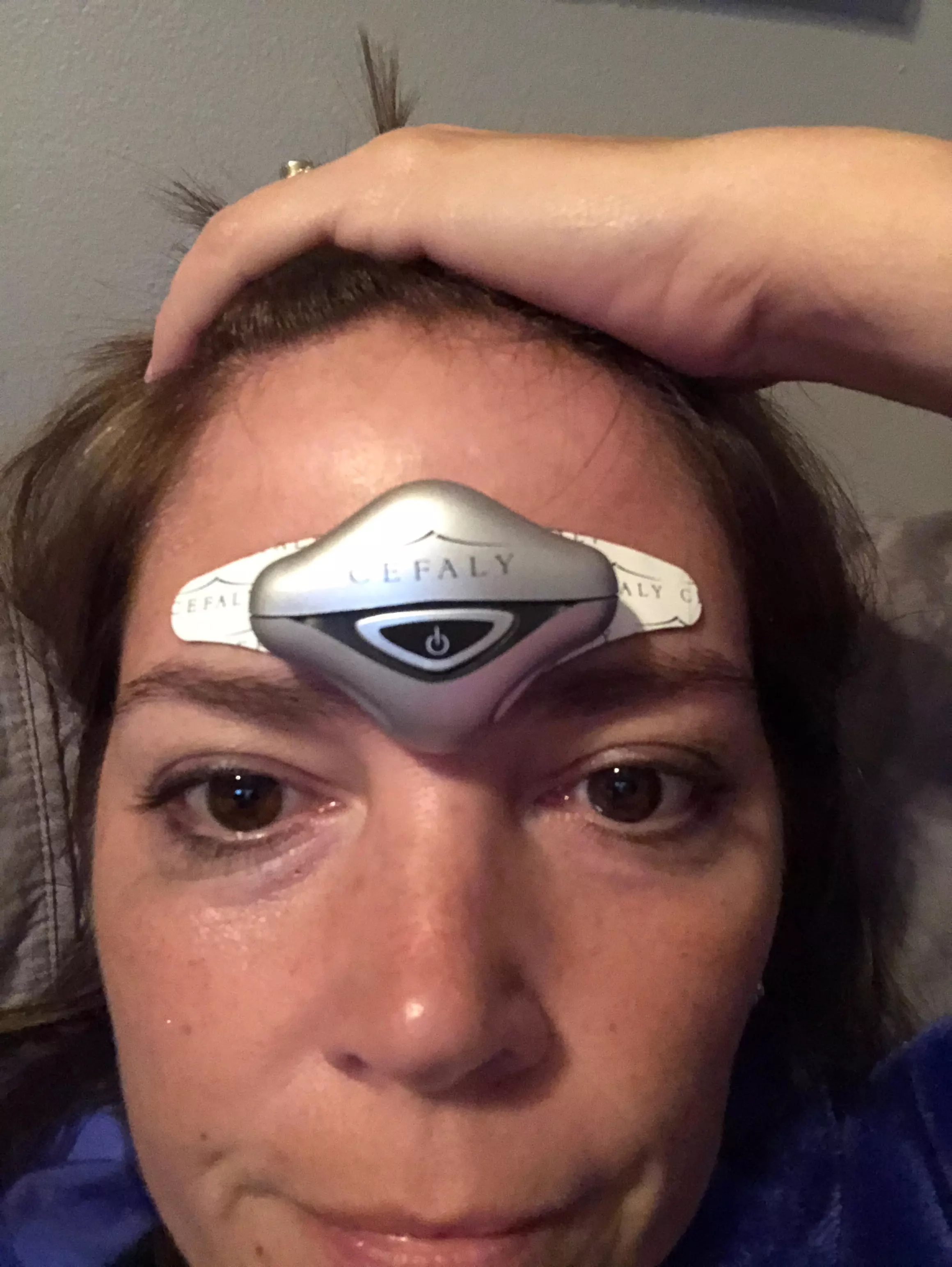 Cefaly A Drug Free Migraine Treatment Prevention Device
Cefaly A Drug Free Migraine Treatment Prevention Device
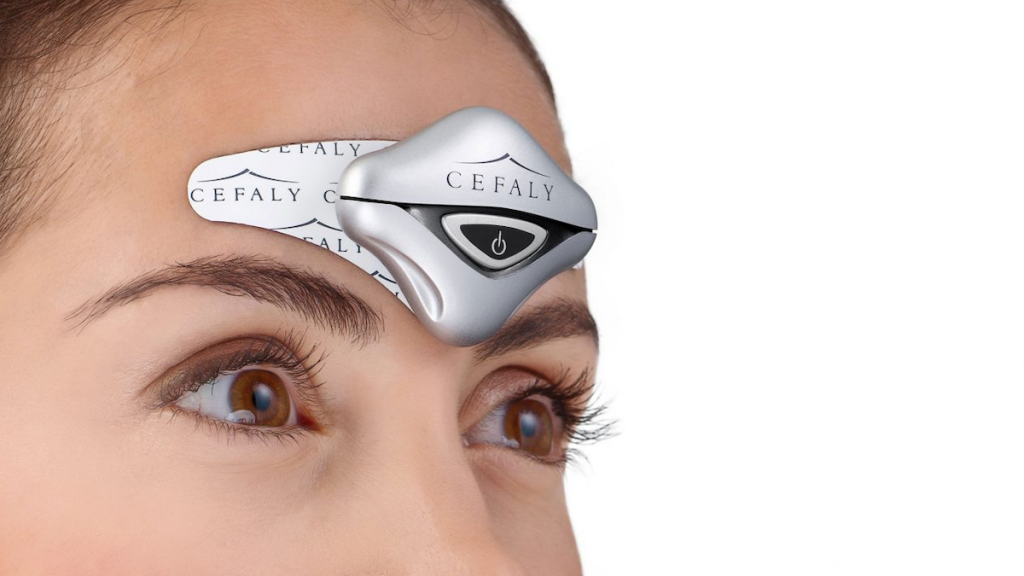 Migraine Canada Cefaly Transcutaneous Stimulation For Migraine
Migraine Canada Cefaly Transcutaneous Stimulation For Migraine
 Cefaly Review For Migraine Does It Work Migrainepal
Cefaly Review For Migraine Does It Work Migrainepal
 Cefaly Review For Migraine Does It Work Migrainepal
Cefaly Review For Migraine Does It Work Migrainepal
 Cefaly Downsizes Migraine Treatment Device Neurology Advisor
Cefaly Downsizes Migraine Treatment Device Neurology Advisor
 Electric Intelligent Mode Migraine Treatment Migraine Device For Headache Buy Migraine Headache Treatment Device Pain Relief Migraine Treatment Pain Relief Migraine Product On Alibaba Com
Electric Intelligent Mode Migraine Treatment Migraine Device For Headache Buy Migraine Headache Treatment Device Pain Relief Migraine Treatment Pain Relief Migraine Product On Alibaba Com
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.