The purpose of study is to evaluate the safety pharmacokinetics and preliminary efficacy of Amivantamab as a monotherapy and in combination with lazertinib and to determine the recommended Phase 2 dose RP2D monotherapy recommended Phase 2 combination dose RP2CD combination therapy and to determine recommended Phase 2 Dose RP2q3W with combination chemotherapy Amivantamab in combination with standard of care carboplatin and pemetrexed in 21 day treatment. Secondary endpoints include safety toxicity objective response rate progression-free survival and overall survival.
 Sec Filing Blueprint Medicines Corp
Sec Filing Blueprint Medicines Corp
The maximum tolerated dose MTD or recommended Phase 2 dose RP2D in this trial was 24 mgm2.

Rp2d clinical trial. The FIHT MAD was tested for eight mAbs 73 in 17 trials 45 of mAbs with available FIHT RP2D. Interventional Clinical Trial Estimated Enrollment. MET abnormalities can drive tumorigenesis.
The study has an expansion portion for PDAC patients at the recommended phase 2 dose RP2D. The RP2D of tepotinib was established as 500 mg once daily. Up to 100 patients will be accrued for this study.
For the q3w schedule n 54 the MTD and RP2D. Of these MTAs 161 64 determined an RP2D. The remaining trials used a nonclassical approach with either multiple endpoints that included toxicity n 87 54 multiple nontoxicity endpoints n 12 7 or a single nontoxicity endpoint n 10 6.
Recommended phase II dose for subsequent clinical trials was only tentatively defined. Primary objective of Phase I study is to determine the recommended phase 2 dose RP2D or maximum tolerated dose MTD in schedule evaluated Assumption. The primary objective of a phase 1 oncology trial is to define the recommended phase 2 dose RP2D of a new drug or multiagent combination in the sched-From the Princess Margaret Cancer Centre University Health Network Division of Medical Oncology and Hematology ARH DMG LLS and the Department of Medicine University of Toronto.
Part 1 was a dose escalation without and with food. In general the recommended 21 phase 2 dose RP2D has been established for an investigational drug or drugs evaluated in a. Tumor mesothelin expression was determined by IHC.
Higher dose greater clinical efficacy Dose-escalation study to determine an acceptable level of dose-limiting toxicity DLT MTDRP2D. Fifty-two trials 32 used toxicity alone to specify an RP2D. - To confirm the safety and anticipated RP2D of REGN2810.
31 ovarian cancer were treated with DMOT4039A. The primary endpoint is to determine the RP2D. - To confirm the safety and anticipated recommended phase 2 dose RP2D of REGN2810 cemiplimab for children with recurrent or refractory solid or Central Nervous System CNS tumors - To characterize the pharmacokinetics PK of REGN2810 given in children with recurrent or refractory solid or CNS tumors Phase 2 Efficacy Phase.
Twenty-nine 16 MTAs were approved by the FDA for solid tumor. The ratio between the doses tested in NFIHTs and the corresponding FIHT RP2Ds. 2015 by American Society of Clinical Oncology INTRODUCTION Theprimaryobjectiveoffirst-in-humanFIHtrials is to determine the safe dose usually known as rec-ommended phase II dose RP2D for the subse-.
Seventy-one patients 40 pancreatic cancer. The objectives of this study were to identify the recommended phase II dose RP2D and assess safety and tolerability pharmacokinetics PK pharmacodynamics PD and antitumor activity of PF-00562271. Antitumor response was evaluated per RECIST 11 and serum CA19-9 or CA125 declines.
The study will include a dose escalation and a dose expansion phase. Phase 1 Dose Escalation to determine Recommended Phase II Dose RP2D of LAE001Prednisone plus Afuresertib in m-CRPC patients. The exploratory endpoints include developing a molecular and immune profile for treatment response.
J Clin Oncol 332158-2165. Trovagene awarded European Commissions Orphan Drug Designation for Onvansertib for treating Acute Myeloid Leukemia in Europe. Part 2 enrolled specific tumor types in an expansion at the RP2D and also assessed the effect of PF-00562271 on.
Once RP2D is determined Phase II will evaluate LAE001Prednisone plus. The purpose of this study is to evaluate the safety and tolerability of ATA188 as a monotherapy in Parts 1 and 2 to determine the recommended Part 2 dose RP2D of ATA188 as monotherapy in Part 1 and to evaluate the effect of ATA188 treatment on clinical disability as assessed by sustained Expanded Disability Status Scale EDSS improvement at 12 months in Part 2 in participants with progressive forms of multiple sclerosis MS primary progressive multiple sclerosis. Up to ten study sites in the United States will participate in the study.
Preclinical data indicated plinabulin has favorable safety and antitumor activity profiles leading to initiation of this clinical trial to determine the recommended phase 2 dose RP2D and assess the safety pharmacokinetics and biologic activity of plinabulin in patients with advanced malignancies. An RP2D of 500 mg once daily as determined from translational modeling and simulation integrating human. This Phase 1 first-in-human open-label multicenter study follows a 33 ascending dose escalation design to determine the MTDRP2D and to characterize the safety tolerability PK and antitumor effects of LNS8801 alone and in combination with pembrolizumab.
20 trial structure master protocols in adult and pediatric cancers. A 33 design was used for dose escalation followed by expansion at the recommended phase II dose RP2D to evaluate safety and pharmacokinetics. This first-in-man trial demonstrated that the potent highly selective MET inhibitor tepotinib can reduce or stabilize tumor burden and is well tolerated at doses up to 1400 mg once daily.
 Are Doses And Schedules Of Small Molecule Targeted Anticancer Drugs Recommended By Phase I Studies Realistic Clinical Cancer Research
Are Doses And Schedules Of Small Molecule Targeted Anticancer Drugs Recommended By Phase I Studies Realistic Clinical Cancer Research
Https Www Ctg Queensu Ca Docs Newinv 2017 Plenarysession1 Phase1clinicaltrialsdesignsandconsiderations Pdf
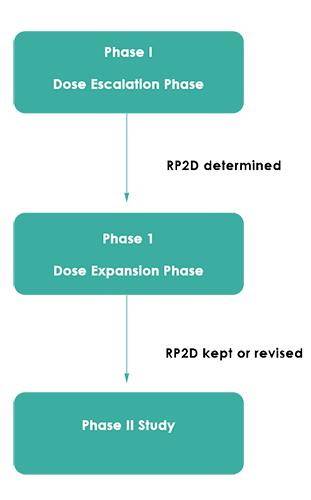 The Expansion Phase Of Phase I Oncology Trials
The Expansion Phase Of Phase I Oncology Trials
 Leticia De Mattos Ar Twitter પર From Aacr20 Clinical Trials Plenary Part 2 Ro7198457 Atezo Phase 1b Trial Pts 4 Lines Prior Therapy Prior Checkpoint In 43 Dose Escalation Majority Pdl1 Low
Leticia De Mattos Ar Twitter પર From Aacr20 Clinical Trials Plenary Part 2 Ro7198457 Atezo Phase 1b Trial Pts 4 Lines Prior Therapy Prior Checkpoint In 43 Dose Escalation Majority Pdl1 Low
 Early Phase Clinical Trials To Identify Optimal Dosing And Safety Sciencedirect
Early Phase Clinical Trials To Identify Optimal Dosing And Safety Sciencedirect
 A Schematic Of The Standard 3 3 Design B Schematic Of The Download Scientific Diagram
A Schematic Of The Standard 3 3 Design B Schematic Of The Download Scientific Diagram
 Patient Flow Diagram Auc Area Under The Curve Rp2d Recommended Download Scientific Diagram
Patient Flow Diagram Auc Area Under The Curve Rp2d Recommended Download Scientific Diagram
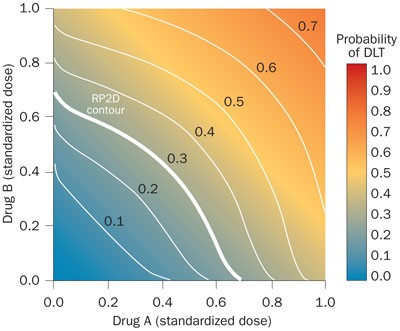 Adaptive Designs For Dual Agent Phase I Dose Escalation Studies Nature Reviews Clinical Oncology
Adaptive Designs For Dual Agent Phase I Dose Escalation Studies Nature Reviews Clinical Oncology
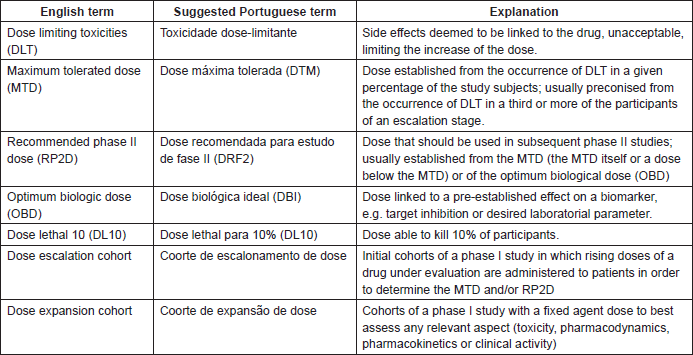 Phase I Trials Of Antitumour Agents Fundamental Concepts Ecancer
Phase I Trials Of Antitumour Agents Fundamental Concepts Ecancer
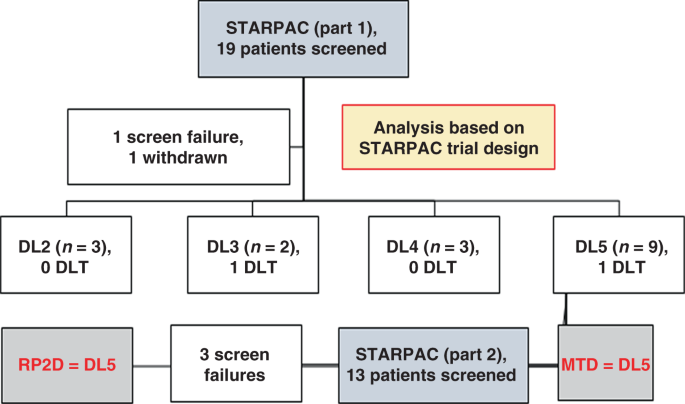 Phase I Clinical Trial Repurposing All Trans Retinoic Acid As A Stromal Targeting Agent For Pancreatic Cancer Nature Communications
Phase I Clinical Trial Repurposing All Trans Retinoic Acid As A Stromal Targeting Agent For Pancreatic Cancer Nature Communications
 Approaches To Phase 1 Clinical Trial Design Focused On Safety Efficiency And Selected Patient Populations A Report From The Clinical Trial Design Task Force Of The National Cancer Institute Investigational Drug Steering
Approaches To Phase 1 Clinical Trial Design Focused On Safety Efficiency And Selected Patient Populations A Report From The Clinical Trial Design Task Force Of The National Cancer Institute Investigational Drug Steering
 A First In Human Phase I Ib Dose Escalation Clinical Trial Of The Autophagy Inducer Abtl0812 In Patients With Advanced Solid Tumours European Journal Of Cancer
A First In Human Phase I Ib Dose Escalation Clinical Trial Of The Autophagy Inducer Abtl0812 In Patients With Advanced Solid Tumours European Journal Of Cancer
 Challenges Of Phase 1 Clinical Trials Evaluating Immune Checkpoint Targeted Antibodies Annals Of Oncology
Challenges Of Phase 1 Clinical Trials Evaluating Immune Checkpoint Targeted Antibodies Annals Of Oncology
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.