And serum ustekinumab concentrations were not impacted by concomitant use of these. Concomitant STELARA was started as part of combination biologic therapy and the patient reported marked improvement after receiving the intravenous induction dose and subsequent maintenance doses.
 Stelara Ustekinumab Mechanism Of Action Psoriatic Arthritis
Stelara Ustekinumab Mechanism Of Action Psoriatic Arthritis
STELARA can be used alone or in combination with methotrexate MTX.

Stelara combination therapy. Dessa läkemedel verkar genom att hämma en del av immunförsvaret. Plackpsoriasis hos vuxna och barn 6 år och äldre. According to the FDA drugs that may interact with Stelara include the following.
Combination therapy with immunomodulators added to the newer biologics ustekinumab Stelara or vedolizumab Entyvio did not improve rates. STELARA alone or in combination with MTX is indicated for the treatment of active psoriatic arthritis in adult patients when the response to previous non-biological disease-modifying anti-rheumatic drug DMARD therapy has been inadequate see section 51. Stelara is a first-in-class biologic therapy.
As is the case with any other medication there may be some instances where Stelara ustekinumab use is not recommended or usage will have to be adjusted in order to prevent or reduce the risk of interactions occurring from other drugs medical conditions or even food and drink. Stelara alone or in combination with methotrexate is indicated for the treatment of active psoriatic arthritis in adult patients when the response to previous non-biological disease-modifying antirheumatic drug DMARD therapy has been inadequate. STELARA is a prescription medicine used to treat adults and children 6 years and older with moderate to severe psoriasis who may benefit from taking injections or pills systemic therapy or phototherapy treatment using ultraviolet light alone or with pills.
1 In patients with CD population pharmacokinetic analyses did not indicate changes in ustekinumab clearance with concomitant use of corticosteroids or immunomodulators AZA 6-MP or MTX. STELARA can be used alone or in combination with methotrexate MTX. STELARA ustekinumab is indicated for the treatment of patients 6 years or older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
My disease is primarily fistulizing and while it hasnt resulted in healing it has kept inflammation in check. Unlike combination therapy with anti-TNF therapy concurrent immunosuppressive therapy does not explain low immunogenicity as only 258 of patients received these and had neither improved clinical outcomes nor higher drug concentrations. The goal afterwards was to eventually discontinue vedolizumab and use STELARA monotherapy to keep the patients Crohns colitis and vulvar Crohns in remission.
Stelara används för att behandla följande inflammatoriska sjukdomar. STELARA can be used alone or in combination with methotrexate MTX. Biologic medications come from natural sources such as the living cells of people plants animals and microorganisms.
Biologics may be successful. The addition of an immunosuppressive drug to Stelara ustekinumab could restore the response to treatment in psoriasis patients according to a study titled Recapturing adequate control of psoriasis by additional immunosuppressive agents alongside ustekinumab published in the Journal of the American Academy of Dermatology Case Reports. When we added Imuran I started at 50mgday and we upped it over time.
Stelara tillhör en grupp läkemedel som kallas immunsuppressiva medel. I am on this mix - Stelara every 4 weeks and 150mg of AzathioprineImuran daily as well as CiproFlagyl. STELARA ustekinumab is indicated for the treatment of patients 6 years or older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
For patients 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy. Dec 11 2019. Use of these concomitant therapies did not appear to influence the overall safety or efficacy of STELARA.
Ustekinumab Stelara intravenous infusion and subcutaneous injection for administration by a healthcare professional FDA Approved Use. Combination Therapy of Apremilast and Biologic Agent as a Safe Option of Psoriatic Arthritis and Psoriasis Apremilast can be safely combined with all biologic agents in patients with plaque psoriasis or psoriatic arthritis not responding adequately to biologics alone. Vad Stelara används för.
STELARA ustekinumab is indicated for the treatment of patients 6 years or older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
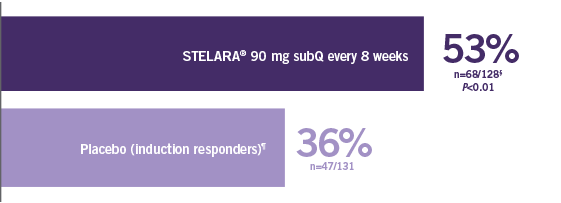 Stelara Ustekinumab Hcp Efficacy Crohn S Disease Cd
Stelara Ustekinumab Hcp Efficacy Crohn S Disease Cd
 Stelara Ustekinumab Efficacy Psoriatic Arthritis
Stelara Ustekinumab Efficacy Psoriatic Arthritis
 Ustekinumab As Induction And Maintenance Therapy For Crohn S Disease Nejm
Ustekinumab As Induction And Maintenance Therapy For Crohn S Disease Nejm
 Learn About Stelara Ustekinumab For Crohn S Disease Stelara
Learn About Stelara Ustekinumab For Crohn S Disease Stelara
 A Review Of Vedolizumab And Ustekinumab For The Treatment Of Inflammatory Bowel Diseases Shim 2018 Jgh Open Wiley Online Library
A Review Of Vedolizumab And Ustekinumab For The Treatment Of Inflammatory Bowel Diseases Shim 2018 Jgh Open Wiley Online Library
Https Www Ema Europa Eu Documents Variation Report Stelara H C 958 Ii 0029 Epar Assessment Report Variation En Pdf
 Why Have We Not Finally Figured Out Combination Therapy
Why Have We Not Finally Figured Out Combination Therapy
 Why Have We Not Finally Figured Out Combination Therapy
Why Have We Not Finally Figured Out Combination Therapy
 Why Have We Not Finally Figured Out Combination Therapy
Why Have We Not Finally Figured Out Combination Therapy
 Stelara Ustekinumab Hcp Efficacy Crohn S Disease Cd
Stelara Ustekinumab Hcp Efficacy Crohn S Disease Cd
 Stelara Ustekinumab Dosing Psoriatic Arthritis
Stelara Ustekinumab Dosing Psoriatic Arthritis
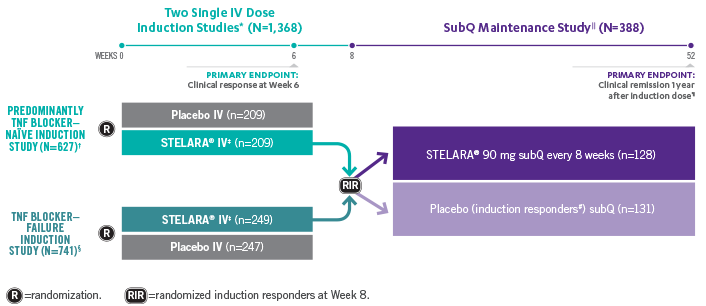 Stelara Ustekinumab Hcp Safety Profile Crohn S Disease Cd
Stelara Ustekinumab Hcp Safety Profile Crohn S Disease Cd
 Stelara Ustekinumab Mechanism Of Action Psoriatic Arthritis
Stelara Ustekinumab Mechanism Of Action Psoriatic Arthritis
 Why Have We Not Finally Figured Out Combination Therapy
Why Have We Not Finally Figured Out Combination Therapy
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.